Lex Genetica: Private-Law Dimension
A priority direction for Russian science is defined in the Decree of the President of the Russian Federation of November 28, 2018 No. 680 “On the development of genetic technologies in the Russian Federation”. The development of genetic technologies will be pursued in accordance with the corresponding Federal Scientific and Technical Program. In order to achieve this goal it will be necessary to develop mechanisms of legal support governing the use of genetic technologies from the stage of scientific research to the implementation of developed results in commercial production. The scientific interest in this problem is due to the currently insufficient legal regulation of relations arising in connection with the use of genetic technologies. However, due to the lack of empirical data, some aspects of the problem must be predicted on the basis of assumptions. Taken together, these problems hinder the active dissemination and implementation of genetic technologies. The purpose of this study is to establish and elucidate issues involved in patenting strains of microorganisms. Microorganism strains represent objects that can be used for scientific and other applications in the context of genetic technologies. Civil law currently permits the patenting of such objects due to their perceived status as an invention. However, despite its objective specificity, there are no additional regulations for governing this process. The concepts of microorganism and strain are presented along with the correct and applicable conditions for their patenting. Examples from foreign experience demonstrate a diversity of national approaches to the procedure for issuing a patent for such inventions. The author offers an opinion regarding the normative consolidation of the analyzed process. The existing Russian regulatory environment features many strong points that can be developed for the patenting of microorganism strains taking into account international experience.
Lex Genetica: Comparative Legal Analysis
The article analyses the experience of key interstate integration associations in relation to regulating the circulation of high-tech (innovative) medicinal products. Trends in the development of supranational regulation in the area under consideration are identified in order to assess its completeness. The relevance of the study is due to the problems faced by both patients and manufacturers of high-tech (innovative) medicinal products: from the patient community’s perspective, there is a problem of access to such expensive drugs, while from the manufacturers’ side, it is necessary to simplify the process of bringing such products to market and ensuring their cross-border circulation. In order to identify trends that may contribute to solving the problems identified by patients and manufacturers, the international legal and supranational framework for regulating the circulation of high-tech drugs is analyzed. The experience of international organizations (in particular, the World Health Organization), as well as key interstate integration associations (the European Union, the African Union, the Eurasian Economic Union (EAEU)), is considered in terms of regulating the circulation of high-tech drugs. The fragmentary and sporadic regulation of the circulation of high-tech drugs at the global level is largely due to the significant differentiation of economic, organizational, and infrastructural capabilities of individual states in relation to the production of such products. The most detailed legal regulation of the circulation of high-tech medical products is implemented within the framework of the European Union, which serves as a benchmark for other interstate integration associations (in particular, the African Union and Eurasian Economic Union). While lacking a formal partnership arrangement with EU structures, the Eurasian Economic Union is de facto implementing its approaches. However, the harmonization and unification of approaches in the area of high-tech drug circulation is hampered by the transfer of a significant number of issues within the EAEU to the national level.
A comparative legal analysis of judicial practice regarding the application of neurotechnologies reveals issues involved in the legal regulation of such novel technologies. The study considers the balance of current legislation in the Russian Federation to determine the need for creating specialized legal regulations for neurotechnologies at both international and national levels. The fragmentary nature of current Russian legislation, which addresses basic issues associated with the introduction of neurotechnologies used exclusively for medical purposes, does not fully reflect their existing potential. As shown by the comparative analysis of the regulatory acts of the Republic of Chile, France, and the USA, the active development of specialized legal regulation is already taking place in these countries. At the international level, UNESCO, UN, and OECD studies and guidelines are aimed at establishing a system of principles and measures to protect existing human rights while recognizing emerging neuro-rights. However, such sof t law instruments and ethical principles have limited impact on national regulators. Thus, due to the specific nature of their impact on human beings and the significant risks of violating individual rights, special regulation of neurotechnologies is necessary at both international and national levels. Such legislative innovations will contribute to establishing a fair balance between technological progress and human rights.
Lex Genetica: Questions of Ethics and Philosophy
The article presents a comprehensive analysis of the ethical aspects of the creation, use, and disposal of medical devices based on nanorobotic technologies. Serious legal and deontological issues arising due to the insuf ficiency of existing ethical and legal norms to deal with rapid technological progress in nanomedicine reveal a dangerous regulatory vacuum. Therefore, the development of fundamental ethical principles to govern the activities of all parties involved throughout the life cycle of nanorobotic systems becomes an urgent priority. For manufacturers, such principles include prioritizing the biocompatibility of materials at both molecular and system levels, ensuring control over targeting and device lifecycle predictability, prioritizing patient well-being over commercial interests, an absolute ban on autonomous decision-making by devices, and clear limits on permissible replication. For medical professionals, the key principles concern obtaining informed consent, ensuring continuous monitoring of the patient’s condition, voluntary use of technology, professional responsibility at all stages of application, empathy, and the mandatory possession of appropriate qualifications. Medical organizations should be guided by the principles of institutional responsibility, including maintenance and compliance with standards, quality assurance, data collection and storage, non-discrimination against patients who have opted out of nanorobotic treatments, as well as the protection of the interests of both patients and medical staff. Ethical principles applying to the recycling of medical devices include a prohibition of reuse and fulfilling environmental safety requirements. The development and implementation of a specialized code of ethics to cover the entire life cycle of medical nanorobotic systems will provide a necessary foundation for the subsequent development of adequate legislative regulations that release the enormous potential of nanorobotics to transform healthcare while ensuring the protection of patients and society.
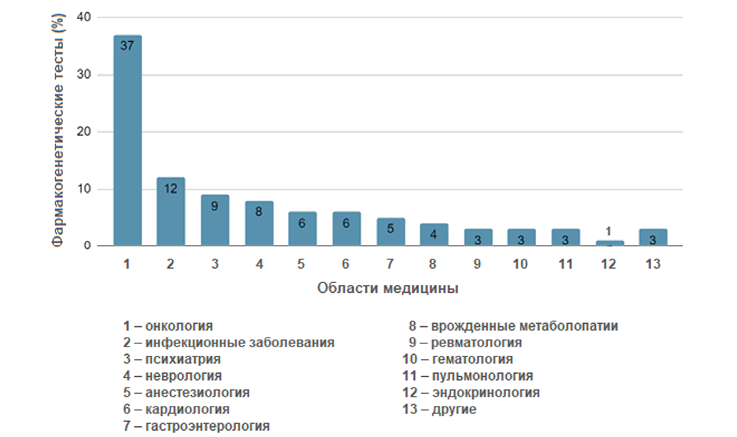
The work presents research into foreign experience of the shif t to personalized psychiatry when taking a psychopharmacogenetic approach. Contemporary trends in the development of genetically oriented treatment of psychiatric disorders are identified in recent international studies. A systematic review of the relevant literature reveals a growing interest in the individualized prescription of drugs based on a patient’s genetic profile as a means of increasing therapeutic ef fectiveness and reducing the risk of side ef fects. In order to evaluate the introduction of psychopharmacogenetics in foreign countries, the review includes data on diagnostic methods, drug prescription protocols, and clinical outcomes. The individual selection of dosages of antidepressants and antipsychotics is shown to have led to a significant increase in the ef fectiveness of treatments for a number of psychiatric diseases. Psychopharmacogenetics thus represents a promising area of modern psychiatry, having the potential to significantly improve the quality of life of patients. However, further clinical trials are needed before introducing appropriate technologies into healthcare practice. Limitations of the study include the small sizes of the study populations and the lack of representativeness of the samples. For this reason, caution should be taken when extrapolating the findings to the general population.
Expert Opinion
Over the past 30 years, over 6,000 human genes have been investigated for their association with the development of central nervous system (CNS) diseases. However, such studies offer poor reproducibility due to methodological differences, heterogeneous and often small sample sizes, as well as the significant influence of genetic variation related to ethnic diversity. Psychiatric disorders may be characterized by their polygenic inheritance, i.e., involving multiple candidate genes. Thus, while the contribution of a specific gene to a particular psychiatric disorder’s pathogenesis is likely to be very small, candidate genes have been identified as partially influencing disease onset. The article sets out fundamental ethical and methodological principles for conducting genetic research and establishing biomaterial repositories – or biobanks. When involving psychiatric patients, such principles must take into account the inherent vulnerability of this group. The conclusions presented in this work may serve as a foundation for developing methodological recommendations and guidelines.
ISSN 3034-1647 (Online)